Increasing Product Reliability
Chugai Group works hard to supply high-quality medicines and pharmaceuticals, and to ensure patients and healthcare providers use its products properly by offering timely and accurate delivery of quality information.
Policy for Regulatory Compliance and Quality Assurance
Chugai’s basic approach is set forth in the policy for regulatory compliance and quality assurance.
The policy for regulatory compliance and quality assurance places specific emphasis on the following four points (four qualities).
- Product quality
- Product information quality
- Business process quality
- Staff suitability
To provide high-quality products and information, Chugai believes it is important to ensure the quality of the business processes that generate them, and to secure suitable personnel to actually perform that work.
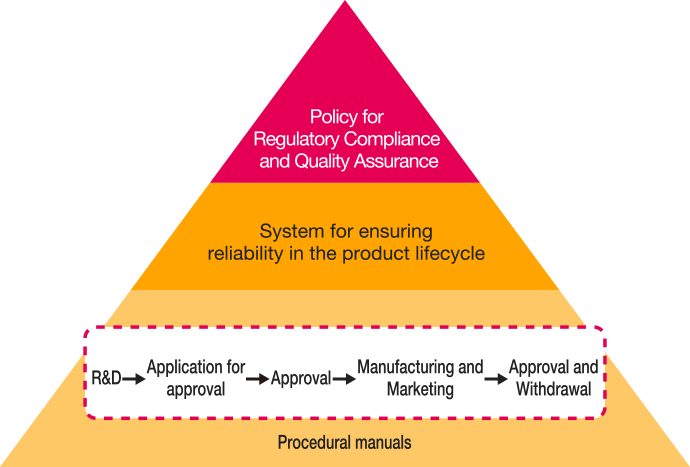
System for Ensuring Reliability in the Product Lifecycle
To provide patients and healthcare workers with products of superior efficacy and safety, Chugai engages in a continuous effort to ensure reliability across the product lifecycle, from R&D to application for approval, manufacturing and marketing, and approval withdrawal.
System for Ensuring Reliability in the Product Lifecycle
Activities to Foster a Culture of Quality
At Chugai, we believe in the importance of an organizational culture that pursues quality by initiating its own improvements in processes and rules. To continue to provide high-quality products, we are engaged in a number of activities designed to foster a culture of quality. We continue to build awareness through dialogue, encouraging our employees to think about what quality means to them and what matters most in improving quality, and ensuring that each of them is able to take independent action.
What Developing Quality Awareness Means
![A diagram showing Chugai’s framework for Conditions that foster an awareness of quality: [Mindset and Attitude] Patients are always put first. Everyone always acts after considering “Why?” rather than following set procedures without question. Everyone takes an interest in quality and works autonomously to continually improve quality. [Communication] Necessary information is conveyed to the right individuals, irrespective of organization or seniority. People speak up without hesitation or fear. Previous mistakes and lessons learned are passed on. [Employee Ownership] All employees see quality as their personal responsibility. All employees work together to meet common quality objectives. [Leadership] Executives and management communicate the importance of quality through quality objectives and a vision. Executives and management take the initiative and set an example for others. Executives and management give credit and support for decisions and actions to improve quality.](/english/sustainability/patient/reliability/images/pic_csr_patient09_2020.png)
Handling of Inquiries by the Medical Information Department
Advances in scientific technology in recent years have seen the development of many innovative drugs and treatment methods. It is necessary to offer the appropriate therapies to patients who need them at the appropriate times. To achieve these goals, it is important that both healthcare providers and patients themselves actively participate in the treatment, and pharmaceutical companies are increasingly required to provide more detailed information in lay terms on product quality, effectiveness, and safety to ensure that drugs are used appropriately.
Chugai’s Medical Information Dept. responds to inquiries from patients, their families, and healthcare providers via telephone, our website, and other diverse channels using resources available to Roche Group members to provide highly specialized and globally consistent information.
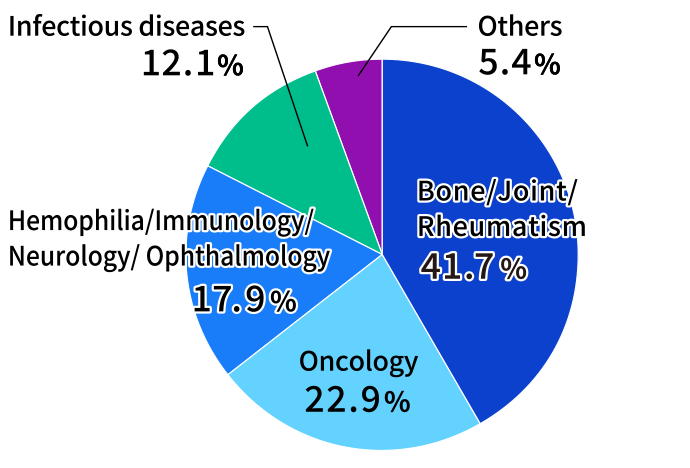
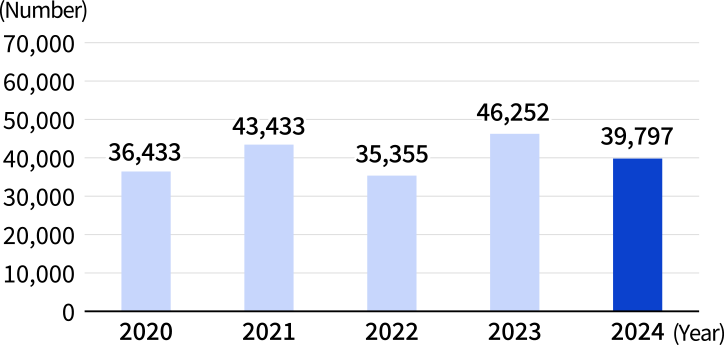
In 2024, the Medical Information Dept. received approximately 40,000 inquiries by phone, email, and other channels. This marked a decrease of about 6,000 from the previous year, partly due to a decline in inquiries related to infectious diseases. About 70% of inquiries came from pharmacists. By drug category, many inquiries were concerning humanized anti-human IL-6 receptor monoclonal antibody preparations, influenza medication, osteoporosis treatments, and anticancer agents. The website for healthcare professionals responds to inquiries by posting Product Q&As and also Web FAQs on the website for patients. In 2024, the Product Q&As on the website for healthcare professionals was browsed approximately 131,000 times, and Web FAQs on the website for patients approximately 78,000 times.
The staff at the center will continue working together to enhance customer satisfaction with a patient-centered philosophy.
Number of Inquiries Received
Inquiries by Treatment Area