Drug Safety Core Themes and Initiatives
Chugai works on the drug safety activities based on the three strategic points: 1) maximization of the value of growth drivers through a commitment to promoting appropriate use, 2) generation of unique evidence to contribute to personalized healthcare (PHC) and building of innovative customer engagement models, and 3) maximization of product value through enhancement of safety measures from the clinical development stage.
Business Model and Core Themes
In Japan and overseas, Chugai handles numerous therapeutic antibodies, molecular targeted therapies, and other pharmaceuticals with innovative modes of action. To promote the appropriate use of these pharmaceuticals around the world and gain acceptance from patients and healthcare providers, Chugai establishes pharmacovigilance protocols with Roche and other partners and collects safety information on a global level.
We consider expert safety evaluation and speedy decision-making throughout the product lifecycle to be essential for timely provision of safety information and implementation of measures to ensure safety. Accordingly, we have established an independent Drug Safety Division and put in place a safety control system directly linked to management. Chugai sees pharmacovigilance activities as part of its mission as a pharmaceutical company and conducts them in an integrated manner, from the collection of safety information to risk assessment and information provision. To ensure the implementation of these activities, all employees receive regular training on how to respond when they receive information on adverse reactions to Chugai products and on matters related to ensuring reliability.
Chugai is committed to continuing with patient-centric activities in this area to build trust, provide truly valuable safety data, and contribute to patients and healthcare worldwide.
Strategy and Progress
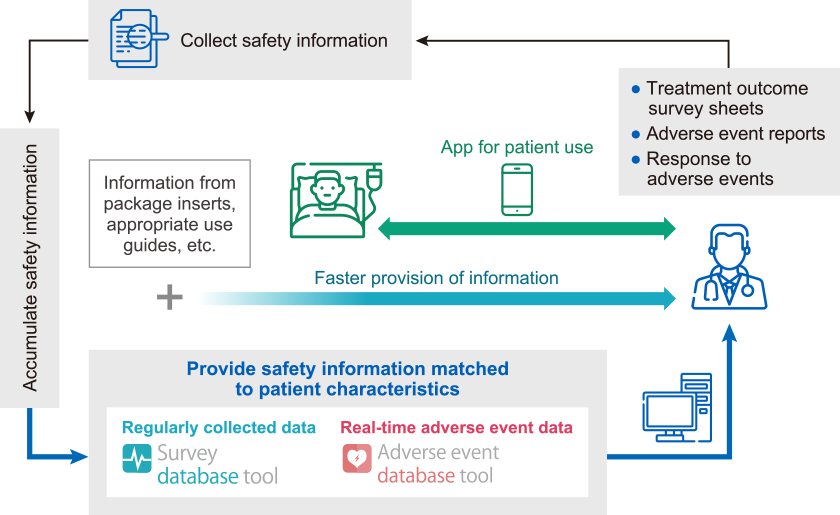
Collecting and managing safety information & risk assessment
With the principal aim of collecting safety information from real-world clinical settings that is unobtainable from clinical trials, we gather reports from medical institutions and data from the literature and scientific conferences. We also collect safety information proactively from post-marketing surveillance, which includes all-case registration surveillance and database surveys. Data on safety collected from medical institutions is analyzed using diverse methods including epidemiology. Risks are assessed to continually evaluate each drug’s safety profile and implement post-marketing drug safety monitoring activities. Information on the results is provided to medical institutions and announced via scientific conferences, papers, and other means. This information is also utilized in drug safety evaluation and safety measures through additional wide-ranging and rigorous management methods, such as management of distribution and confirmation of conditions of use, for numerous anticancer agents, innovative new biopharmaceuticals, and other drugs.
Risk management based on pharmaceutical RMPs
Chugai reflects risk assessment results in risk management plans (RMPs), has been ahead of its competitors in drawing up and applying RMPs, and discloses them on its website. Chugai considers RMPs to be part of its commitment to patients and healthcare professionals. In applying RMPs, we believe we need to strengthen our ability to analyze data from an epidemiological standpoint. This is another area where we have led the industry. To help upgrade Japan’s epidemiological database, a group of Chugai experts with responsibility for epidemiological functions has collaborated with specialized enterprises and other bodies in a range of active initiatives, including formulating recommendations and guidance on database research for presentation to the regulatory authorities. We have launched an initiative to apply database research at the practical level to pharmacovigilance for Chugai products in order to ensure swift and effective collection of safety information, which will enhance and strengthen the operation of RMPs. We have also established procedures for responding to emergencies, such as product recalls, in the event that our risk assessments identify a safety concern, and these processes are checked regularly.
Communications on safety
Chugai provides information on noteworthy adverse events to medical institutions and academic societies. We also distribute information leaflets for patients, post information on our website, and present a variety of lectures in an effort to improve understanding of drug safety and promote appropriate use. In particular, our ability to rapidly provide information according to patient characteristics through the post-marketing surveillance database tool (PMS DB tool) and safety information database tool (SAFETY DB tool) that we began operating in 2016 has won praise from healthcare professionals. With these tools, which include domestic post-marketing safety data (approximately 300,000 cases as of November 2021), we can provide safety information in a timely manner to respond to urgent needs. Starting in 2018, we have additionally rolled out a clinical studies database tool that presents global clinical study safety data submitted for regulatory approval, allowing healthcare professionals to use new products with confidence from immediately after their sales release. In May 2020, we launched an adverse event database tool on our dedicated website for healthcare professionals. This has further broadened our contribution to patient treatment by putting in place a system that enables healthcare professionals to directly access adverse event information on Chugai products whenever needed.
At Chugai, we are also concerned with ensuring smooth communication between patients and healthcare professionals so that patients can undergo treatment free of anxiety. In October 2020, in a joint project with Welby Inc., we began operating a patient support program based on Welby MyKarte ONC, a free personal health record (PHR) service provided by our project partner. This program, designed for cancer patients being treated with the immune checkpoint inhibitor, performs simple integrated management of information on day-to-day symptoms and treatment status to provide information optimized in line with the type of cancer and the symptoms. These functions support patients to achieve a better understanding of their treatment and are also designed to lead to better outcomes by keeping healthcare professionals constantly informed of the patient’s condition, including adverse events and worsening of symptoms.
Cycle of Safety Information Collection, Accumulation, and Provision at Chugai