Chugai News Releases are issued to provide stakeholders with the most up-to-date information related to our company. In some instances, information on products or drug candidates under development may be included, but this is intended for members of the media, shareholders, and investors. The information is not intended for promotional or advertising purposes, or as medical advice, etc.
Jan 29, 2026
- Corporate
Chugai Announces 2025 Full Year Results and Forecasts for 2026
- Record-high core revenue, core operating profit and core net income for the fiscal year 2025 at ¥1,257.9 billion (+7.5%), ¥623.2 billion (+12.1%) and ¥451.0 billion (+13.6%) respectively, with a high core operating profit margin of 49.5% (all changes year-on-year [YoY])
-
Steady progress in R&D activities for both early and late-stage development. For Chugai-originated developments, NXT007, an in-house project, achieved Proof of Concept (PoC), an important milestone, and Eli Lilly, the out-licensing partner, submitted a New Drug Application to the U.S. FDA for orforglipron for the treatment of obesity based on favorable Phase III clinical trial results.
-
Core revenue and core operating profit in 2026 are expected to reach record highs of ¥1,345.0 billion (+6.9%, YoY) and ¥670.0 billion (+7.5%, YoY), respectively
TOKYO, January 29, 2026 -- Chugai Pharmaceutical Co., Ltd. (TOKYO: 4519) announced its consolidated financial results for the fiscal year ended December 31, 2025, and forecasts for the fiscal year ending December 31, 2026.
<Full year core results for 2025>
Chugai reported that revenue for the fiscal year ended December 31, 2025 totaled ¥1,257.9 billion (+ ¥87.3 billion, +7.5%, YoY).
Regarding revenue, domestic sales were ¥472.4 billion (+ ¥11.3 billion, +2.5%, YoY). In the oncology field, sales decreased by 0.5% YoY due to the decrease in Perjeta, which has the same active ingredient as Phesgo, due to Phesgo's market penetration, and the impact of NHI drug price revisions and penetration of biosimilars on our mainstay product Avastin, despite steady growth of new products Phesgo and Lunsumio, and our mainstay product Polivy. In the specialty field, sales increased by 5.8% YoY, driven by steady performance of mainstay products Vabysmo, Enspryng, and Hemlibra, along with successful market penetration of the new product PiaSky. Overseas sales increased by 12.8%, driven by increases in Hemlibra and Actemra to Roche. Other revenue increased by 4.3%, despite the decrease in one-time income, mainly due to an increase in income related to Hemlibra.
Cost to sales ratio improved by 1.3 percentage points YoY to 32.6%, mainly due to foreign exchange effects and changes in the product mix. Research and development expenses increased to ¥180.1 billion (+1.8%, YoY) due to investments into drug discovery and early development, and increases associated with the progress of development projects, while selling, general and administrative expenses were ¥103.2 billion (+1.0%, YoY) mainly driven by miscellaneous expenses. Other operating income (expense) was ¥0.0 billion. As a result, core operating profit was ¥623.2 billion (+12.1%, YoY) with a core operating profit margin (to revenue) of 49.5%, and core net income increased for the nine consecutive years to ¥451.0 billion (+13.6%, YoY).
<R&D activities>
Chugai made steady progress in R&D activities for both in-house products and products in-licensed from Roche.
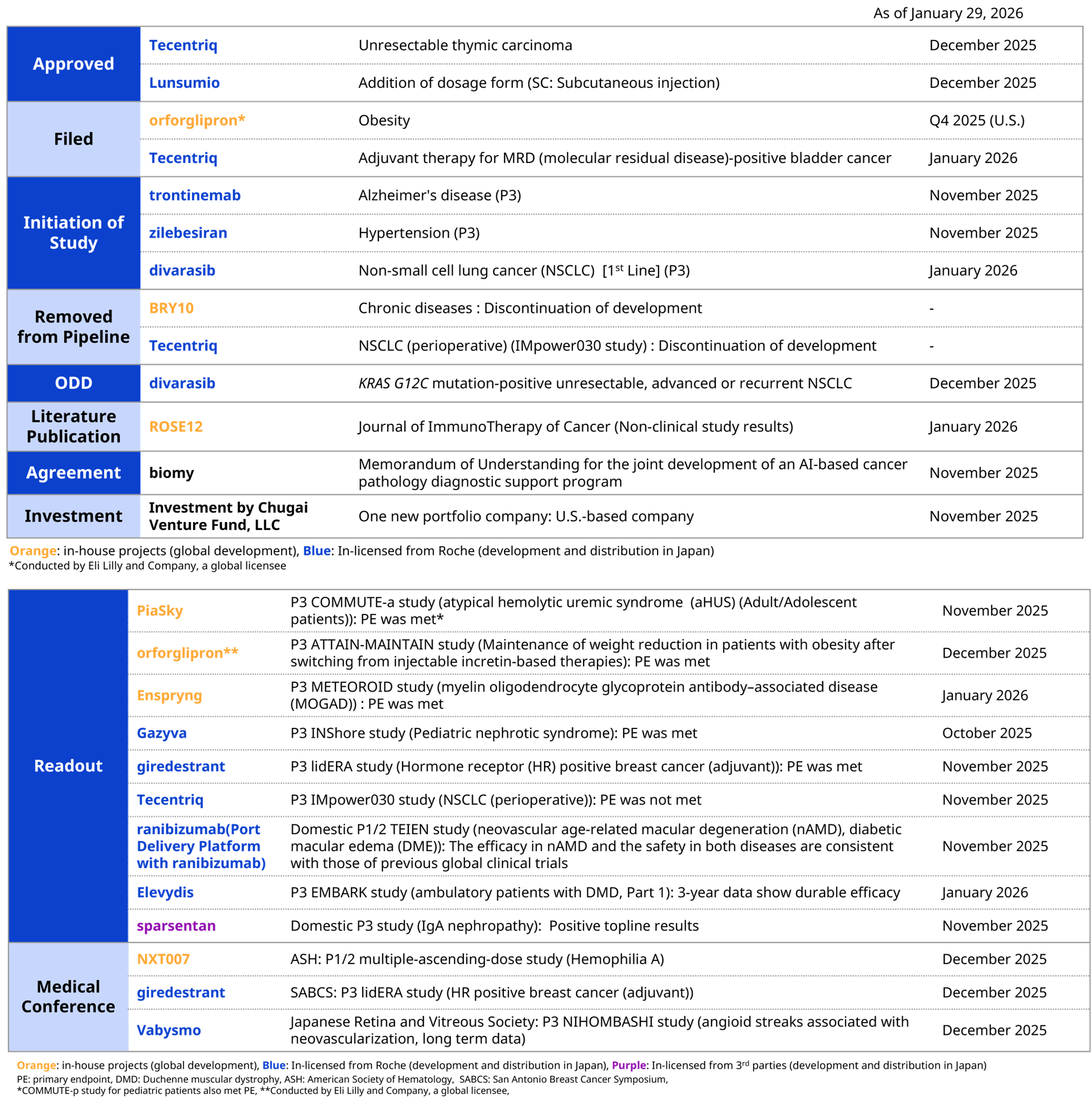
For in-house projects, NXT007, under development for hemophilia A, achieved Proof of Concept (PoC), an important milestone, with data from the high-dose cohort of the Phase I/II trial suggesting the potential to provide blood coagulation capacity equivalent to normal levels. For a Chugai-originated development, orforglipron, an oral GLP-1 receptor agonist out-licensed to Eli Lilly and Company, met the primary endpoints across all multiple Phase III clinical trials for obesity and type 2 diabetes. Based on favorable Phase III clinical trial results, Eli Lilly and Company submitted a New Drug Application to the U.S. Food and Drug Administration (FDA) for the treatment of obesity. NEMLUVIO, being developed overseas by Galderma, received approval in Europe for moderate to severe atopic dermatitis and prurigo nodularis. AVMAPKI, out-licensed to Verastem Oncology, was approved by the FDA under the accelerated approval pathway based on response rate and duration of response for combination therapy with FAKZYNJA for KRAS-mutant recurrent low-grade serous ovarian cancer.*
*Continued approval may be contingent upon verification and description of clinical benefit in confirmatory trials.
For products in-licensed from Roche, the intravenous formulation of Lunsumio was launched in Japan as a treatment for relapsed or refractory follicular lymphoma, and additional approval was obtained for the subcutaneous formulation. In addition, Elevidys obtained regulatory approval in Japan as a regenerative medicine product for the treatment of duchenne muscular dystrophy under the conditional and time-limited approval pathway. Furthermore, Tecentriq and Vabysmo obtained approvals for expanded indications, and Lunsumio and Avastin have been filed for expanded indications.
For products in-licensed from third parties other than Roche, sparsentan, acquired through the wholly owned subsidiary of Renalys Pharma, Inc., showed positive topline results in a domestic Phase III trial for IgA nephropathy. Sparsentan is scheduled to be filed for regulatory approval in Japan in 2026.
In 2025, Chugai entered into a joint research and license agreement with Gero, which has target discovery technology for age-related diseases, and a license agreement with Rani Therapeutics, whose technology enables oral delivery of biologics and drugs. Chugai will continue to pursue the expansion of its drug discovery engine through open innovation.
<Full year forecast for 2026>
In 2026, core revenue, core operating profit, and core net income are expected to be ¥1,345.0 billion (+ ¥87.1 billion, +6.9%, YoY), ¥670.0 billion (+ ¥46.8 billion, +7.5%, YoY), and ¥485.0 billion (+ ¥34.0 billion, +7.5%, YoY), resulting in an increase in both revenue and profit. Product sales are expected to increase in Japan and remain at similar levels to the previous year overseas, totaling ¥1,100.0 billion (+ ¥22.2 billion, +2.1%, YoY). Domestic sales are expected to be ¥498.0 billion (+ ¥25.6 billion, +5.4%, YoY) due to volume growth of new product Lunsumio and our mainstay products, despite a decrease in sales due to NHI drug price revisions and penetration of generics. Overseas sales are expected to be ¥602.0 billion (- ¥3.4 billion, -0.6%, YoY) due to growth in NEMLUVIO and Hemlibra, while a decrease in Actemra and others is expected. Other revenue is expected to be ¥245.0 billion (+ ¥64.9 billion, +36.0%, YoY). Royalty and profit-sharing income are forecasted to be ¥217.2 billion (+ ¥44.5 billion, +25.8%, YoY), due to an increase in income related to products out-licensed to third parties and Hemlibra. Other operating income is expected to be ¥27.8 billion (+ ¥20.3 billion, +270.7%, YoY), due to an increase in one-time income.
[2025 full year results]
| Billion JPY | 2025 | 2024 | % change |
|---|---|---|---|
| Core results | |||
| Revenue | 1,257.9 | 1,170.6 | +7.5% |
| Sales | 1,077.8 | 997.9 | +8.0% |
| Other revenue | 180.1 | 172.7 | +4.3% |
| Operating profit | 623.2 | 556.1 | +12.1% |
| Net income | 451.0 | 397.1 | +13.6% |
| IFRS results | |||
| Revenue | 1,257.9 | 1,170.6 | +7.5% |
| Operating profit | 598.8 | 542.0 | +10.5% |
| Net income | 434.0 | 387.3 | +12.1% |
[Sales breakdown]
| Billion JPY | 2025 | 2024 | % change |
|---|---|---|---|
| Sales | 1,077.8 | 997.9 | +8.0% |
| Domestic sales | 472.4 | 461.1 | -0.5% |
| Oncology | 246.5 | 247.7 | -0.5% |
| Specialty | 225.8 | 213.4 | +5.8% |
| Overseas sales | 605.4 | 536.8 | +12.8% |
[Oncology field (Domestic) Top5-selling medicines]
| Billion JPY | 2025 | 2024 | % change |
|---|---|---|---|
| Tecentriq | 62.8 | 65.4 | -4.0% |
| Polivy | 37.2 | 34.1 | +9.1% |
| Phesgo | 33.9 | 23.5 | +44.3% |
| Alecensa | 33.5 | 31.0 |
+8.1% |
| Avastin | 26.1 | 33.8 | -22.8% |
[Specialty field (Domestic) Top5-selling medicines]
| Billion JPY | 2025 | 2024 | % change |
|---|---|---|---|
| Hemlibra | 62.7 | 59.0 | +6.3% |
| Actemra | 50.5 | 48.0 | +5.2% |
| Enspryng | 29.2 | 24.7 | +18.2% |
| Vabysmo | 26.2 | 21.5 | +21.9% |
| Evrysdi | 16.2 | 15.9 | +1.9% |
[2026 full year forecast](Core-basis)
| Billion JPY | 2026 Forecast | 2025 Actual | % change |
|---|---|---|---|
| Revenue | 1,345.0 | 1,257.9 | +6.9% |
| Operating profit | 670.0 | 623.2 | +7.5% |
| Net income | 485.0 | 451.0 | +7.5% |
[Progress in R&D activities from Oct 25th, 2025 to Jan 29th, 2026]
About Core results
Chugai discloses its results on a Core basis from 2013 in conjunction with its decision to apply IFRS. Core results are the results after adjusting Non-Core items to IFRS results. Chugai’s recognition of non-recurring items may differ from that of Roche due to the difference in the scale of operations, the scope of business and other factors. Core results are used by Chugai as an internal performance indicator, for explaining the underlying business performance both internally and externally, and as the basis for payment-by-results such as a return to shareholders.
Trademarks used or mentioned in this release are protected by law.
Contact:
- For Media
- Chugai Pharmaceutical Co., Ltd.
- Media Relations Group, Corporate Communications Dept.,
- Hideki Sato
- Tel: +81-3-3273-0881
- E-mail: pr@chugai-pharm.co.jp
- For Investors
- Chugai Pharmaceutical Co., Ltd.
- Investor Relations Group, Corporate Communications Dept.,
- Takayuki Sakurai
- Tel: +81-3-3273-0554
- E-mail: ir@chugai-pharm.co.jp