Chugai News Releases are issued to provide stakeholders with the most up-to-date information related to our company. In some instances, information on products or drug candidates under development may be included, but this is intended for members of the media, shareholders, and investors. The information is not intended for promotional or advertising purposes, or as medical advice, etc.
Oct 25, 2024
- Corporate
Chugai Announces 2024 3rd Quarter Results
- Core revenue, core operating profit, and core net income at ¥868.5 billion (+3.7%), ¥426.6 billion (+25.3%), and ¥301.3 billion (+20.4%), respectively (all changes year-on-year)
- Both revenue and profit increased, led by strong overseas exports
- Forecasts for FY2024 revised upward
- Research and development activities have been progressing steadily in both early and late stage developments
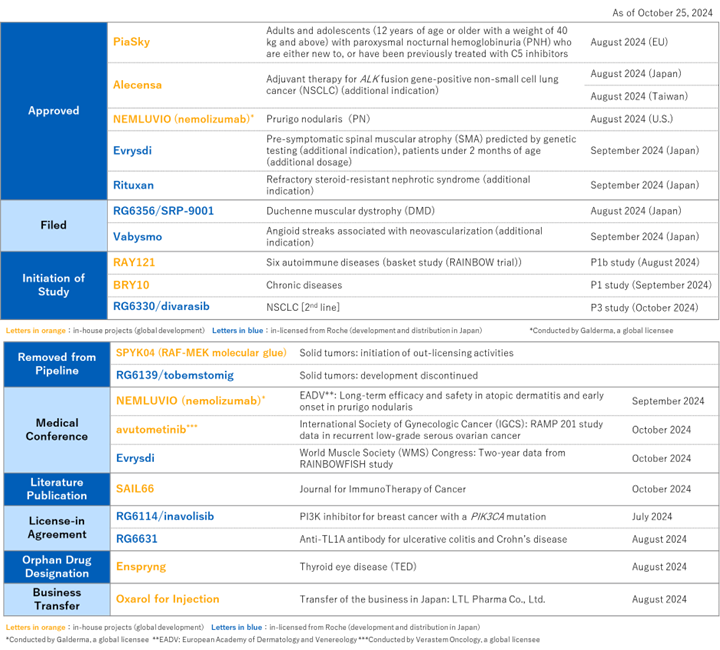
- For in-house products, PiaSky has been approved in Europe, and NEMLUVIO, out-licensed to Galderma, has been approved in the U.S. Alecensa was approved for additional indication in Japan
- For in-house early-stage projects, RAY121 has initiated a basket trial aiming for simultaneous development in six diseases
- For in-licensed products, we achieved filing for regulatory approval of gene therapy product delandistrogene moxeparvovec and Vabysmo, as well as the expansion of indication for Evrysdi
TOKYO, October 25, 2024 -- Chugai Pharmaceutical Co., Ltd. (TOKYO: 4519) announced its financial results for the third quarter of fiscal year 2024.
“In the third quarter of 2024, we achieved increased revenue and profit, primarily driven by strong overseas exports. While the domestic sales continued to be affected by the completion of government supply of Ronapreve® in the previous year, as well as the impact of biosimilars and NHI drug price revisions, our overseas revenue growth was led by a significant increase in exports of our in-house developed product Hemlibra® to Roche. In consideration of the good progress as of the third quarter, we raised our full year forecasts for FY2024. In research and development, PiaSky® received approval in Europe for paroxysmal nocturnal hemoglobinuria (PNH), while NEMLUVIO®(nemolizumab), out-licensed to Galderma, was approved in the United States for prurigo nodularis. Furthermore, Alecensa® obtained approval for additional indication in Japan for adjuvant therapy for ALK-positive non-small cell lung cancer. We are very pleased that these in-house developed products can contribute to the treatment of more patients. In early-stage development, our in-house project RAY121 initiated a phase Ib basket trial targeting six autoimmune diseases, aiming for early value maximization. This basket trial approach outside the oncology field is a novel challenge with few precedents worldwide. We will continue to pursue innovation to deliver innovative new drugs to patients around the world,” said Dr. Osamu Okuda, Chugai’s President and CEO.
<Third Quarter Financial Results (Core results, January to September 2024)>
Chugai reported increased revenue by 3.7% and operating profit by 25.3% year-on-year for the third quarter (nine months, Core-basis), mainly driven by strong overseas exports, and increase in other revenue.
Regarding revenue, domestic sales decreased by 22.7%. In the oncology field, although the growth of new product Phesgo® was favorable, the overall decrease was 5.8% due to Avastin® biosimilars and other impacts. In the specialty field, sales decreased by 36.4%, mainly due to continued impact of the completion of Ronapreve supply to the government in the previous year, while our new product Vabysmo® grew and PiaSky was launched positively, and our mainstay product Actemra® performed well. Overseas, Hemlibra’s exports to Roche increased greatly by 47.9%, leading to the increase by 33.8% in total overseas sales. Our mainstay product Alecensa also performed well, and Enspryng® almost doubled its sales compared to the previous year. Other revenue increased by 23.8%, driven by the increase in Hemlibra related income including royalties, and one-time income.
Cost to sales ratio improved by 10.6 percentage points year-on-year to 32.5%, mainly due to a change in the product mix. Research and development expenses increased by 5.1% mainly due to investments into drug discovery and early development, and increases associated with the progress of development projects. Selling, general and administration expenses increased by 1.5% mainly due to foreign exchange rate fluctuations and an increase in the enterprise tax (pro forma standard taxation). Other operating income (expense) was ¥2.4 billion in income, including the recognition of income from disposal of product rights. As a result, Core operating profit totaled ¥426.6 billion (+25.3%).
<R&D activities>
Chugai also made good progress in research and development, in both early and late stages of developments.
For in-house products, Alecensa has been granted additional indication in Japan and Taiwan for adjuvant treatment of ALK-positive non-small cell lung cancer (NSCLC). Additionally, PiaSky, an anti-complement (C5) recycling antibody, has been approved in Europe for the treatment of paroxysmal nocturnal hemoglobinuria (PNH). It is the first monthly (every four weeks) subcutaneous treatment for PNH in Europe. Furthermore, NEMLUVIO, developed by Galderma outside of Japan, has been approved under priority review in the U.S. as the treatment of adults with prurigo nodularis. In early development, RAY121, an anti-complement C1s recycling antibody, has initiated a phase Ib clinical study as a basket study in six autoimmune diseases. Moreover, BRY10 has initiated a phase I clinical study for chronic diseases and has been added to the pipeline.
For the products in-licensed from Roche, delandistrogene moxeparvovec, a gene therapy product (development code: SRP-9001, overseas product name: Elevidys) has been filed for Duchenne muscular dystrophy. Additionally, Vabysmo has been filed for an additional indication for angioid streaks associated with neovascularization. Evrysdi® has been approved for an additional indication for pre-symptomatic spinal muscular atrophy (SMA) and an additional dosage for SMA infants under 2 months of age. Furthermore, divarasib, the KRAS G12C inhibitor, has initiated a phase III clinical study for second-line treatment of NSCLC. In addition, Chugai has in-licensed the PI3K inhibitor inavolisib for PIK3CA-mutated breast cancer and the anti-TL1A therapy RG6631 for refractory diseases such as ulcerative colitis and Crohn's disease.
<Revision of Full-Year Forecast for 2024>
Chugai raised forecasts (Core-basis) for FY2024 following the strong nine-month results. Regarding domestic sales, the forecasted sales amount reflects the progress and revised assumptions for products including Phesgo, Polivy®, Vabysmo, and Perjeta®. For overseas sales, mainly Hemlibra and Actemra exports to Roche have been forecasted to be higher than the original forecast. For other revenue, the forecasts for items including one-time income and royalties have also been updated. As a result, revenue has been raised to ¥1,150.0 billion, an increase of ¥80.0 billion from the initial forecast. Operating profit forecast has been revised to ¥540.0 billion, up ¥80.0 billion from the initial forecast, taking into account a lower cost to sales ratio due to a change in the product mix from the initial assumption, and increase in some expenses.
Reflecting the significant changes in the business environment, year-end dividend forecast has been revised to undecided. The year-end dividends will be decided after the fiscal year end based on basic profit distribution principles*.
*Regarding income distribution, taking into account the strategic funding needs and earning prospects, Chugai aims for a consolidated dividend payout ratio of 45% on average in comparison with Core EPS to provide a stable allocation of profit to all shareholders.
[2024 third quarter results]
| Billion JPY | 2024 Jan - Sep | 2023 Jan - Sep | % change |
|---|---|---|---|
| Core results | |||
| Revenue | 868.5 | 837.6 | +3.7% |
| Sales | 750.3 | 742.1 | +1.1% |
| Other revenue | 118.2 | 95.5 | +23.8% |
| Operating profit | 426.6 | 340.5 | +25.3% |
| Net income | 301.3 | 250.3 | +20.4% |
| IFRS results | |||
| Revenue | 868.5 | 837.6 | +3.7% |
| Operating profit | 418.6 | 317.6 | +31.8% |
| Net income | 295.8 | 234.3 | +26.2% |
[Sales breakdown]
| Billion JPY | 2024 Jan - Sep | 2023 Jan - Sep | % change |
|---|---|---|---|
| Sales | 750.3 | 742.1 | +1.1% |
| Domestic sales | 331.7 | 429.2 | -22.7% |
| Oncology | 180.3 | 191.4 | -5.8% |
| Specialty | 151.3 | 237.9 | -36.4% |
| Overseas sales | 418.7 | 312.9 | +33.8% |
[Oncology field (Domestic) Top5-selling medicines]
| Billion JPY | 2024 Jan - Sep | 2023 Jan - Sep | % change |
|---|---|---|---|
| Tecentriq | 47.4 | 47.9 | -1.0% |
| Avastin | 25.6 | 38.2 | -33.0% |
| Polivy | 24.5 | 25.5 | -3.9% |
| Alecensa | 22.4 | 22.0 | +1.8% |
| Perjeta | 15.7 | 24.6 | -36.2% |
[Specialty field (Domestic) Top5-selling medicines plus Ronapreve]
| Billion JPY | 2024 Jan - Sep | 2023 Jan - Sep | % change |
|---|---|---|---|
| Hemlibra | 41.5 | 40.5 | +2.5% |
| Actemra | 34.8 | 32.2 | +8.1% |
| Enspryng | 17.8 | 16.9 | +5.3% |
| Vabysmo | 14.7 | 10.8 | +36.1% |
| Evrysdi | 11.3 | 10.3 | +9.7% |
| Ronapreve* | - | 81.2 | -100.0% |
*Ronapreve has not been listed in the National Health Insurance (NHI) price list.
[Forecasts for 2024 Jan-Dec]
| Billion JPY | Revised forecast on Oct 25 | Original forecast on Feb 1 | Growth vs original forecast | Growth vs 2023 actual |
|---|---|---|---|---|
| Core-basis | ||||
| Revenue | 1,150.0 | 1,070.0 | +7.5% | +38.6, +3.5% |
| Sales | 986.0 | 922.0 | +6.9% | +11.5, +1.2% |
| Other revenue | 164.0 | 148.0 | +10.8% | +27.1, +19.8% |
| Operating profit | 540.0 | 460.0 | +17.4% | +89.3, +19.8% |
[Progress in R&D activities from Jul 26th, 2024 to Oct 25th, 2024]
About Core results
Chugai discloses its results on a Core basis from 2013 in conjunction with its decision to apply IFRS. Core results are the results after adjusting non-Core items to IFRS results. Chugai’s recognition of non-recurring items may differ from that of Roche due to the difference in the scale of operations, the scope of business and other factors. Core results are used by Chugai as an internal performance indicator, for explaining the underlying business performance both internally and externally, and as the basis for payment-by-results such as a return to shareholders.
Trademarks used or mentioned in this release are protected by law.
Contact:
- For Media
- Chugai Pharmaceutical Co., Ltd.
- Media Relations Group, Corporate Communications Dept.,
- Hideki Sato
- Tel: +81-3-3273-0881
- E-mail: pr@chugai-pharm.co.jp
- For Investors
- Chugai Pharmaceutical Co., Ltd.
- Investor Relations Group, Corporate Communications Dept.,
- Takayuki Sakurai
- Tel: +81-3-3273-0554
- E-mail: ir@chugai-pharm.co.jp
