May 30, 2023
- Corporate
New Investment of Over 50 Billion Yen in Bio Manufacturing Facilities to Strengthen the Production Base to Support the Rapid Launch of In-House Projects
- Decided to invest over 50 billion yen in total in 2 new manufacturing facilities at Utsunomiya Plant
- Strengthen the in-house foundation to manufacture bio-APIs, from clinical development stage to initial commercial production, by establishing a new facility for middle to later- stage manufacturing of investigational drugs including phase 1 and 2 projects, and initial commercial products
- Construction of a new injection building to support the formulation of complex in-house antibody projects and utilize robotics to support the use of various containers and to improve productivity
TOKYO, May 30, 2023 -- Chugai Pharmaceutical Co., Ltd. (TOKYO: 4519) announced its decision today to construct a new bio active pharmaceutical ingredients (APIs) manufacturing building and injection building in the Utsunomiya Plant (Utsunomiya City, Tochigi Prefecture) of Chugai Pharma Manufacturing Co., Ltd. (Head office: Tokyo, President: Shinya Takuma), a member of the Chugai Group.
The new bio-API manufacturing building (UT3) will target the clinical development stage to initial commercial production by establishing a new facility for middle to later-stage manufacturing of investigational drugs, including Phase 1 and 2 projects. Together with the bio-API manufacturing building for early-stage clinical development (UK4), currently under construction at the Ukima Site, and existing manufacturing buildings, investment in UT3 will further strengthen the in-house supply foundation from clinical development to initial production, thereby contributing to the rapid launch of in-house products. UT3 will also implement continuous production functions by introducing perfusion culture*, in addition to the conventional batch-type production system, promoting initiatives to realize the concept of a next-generation biopharmaceutical factory. The new injection building (UTA) will be responsible for manufacturing sterile injectables for initial commercial use. New formulation technology will be introduced to support the formulation of complex structured antibodies using Chugai’s proprietary antibody engineering technologies. Furthermore, using robotics will enable high-mix, low-volume manufacturing in response to a wide variety of dosage forms that will lead to patient convenience. At the same time, we aim to realize smart factories which substantially enhance productivity by utilizing advanced automatic driving technology and other digital technologies.
*A cell culture method in which antibodies are harvested from a culture vessel that has antibody-producing cells maturing at high density under a continuous nutrient supply. It promises improved production efficiency compared to the conventional method, where antibodies are not collected until the culture is terminated.
“With the adoption of Chugai’s proprietary antibody engineering technologies, which the company has its strength in, the complexity of manufacturing such antibodies is increasing. Securing the technology and capacity to respond to such difficult projects and enable in-house manufacturing from the clinical development stage to initial commercial production will bring speed and flexibility to the development of in-house projects, leading to the acquisition of a significant competitive advantage,” said Chugai’s President and CEO, Dr. Osamu Okuda. “In addition to pursuing sustainability, including consideration for the environment, we will also use digital technologies and robotics to increase productivity dramatically and realize world-class pharmaceutical and manufacturing functions suitable for a top innovator in the healthcare industry. We are committed to ensuring that innovative drug discovery ideas from our research function are realized as pharmaceuticals and to contribute to patients around the world.”
The new manufacturing facilities, UT3 and UTA, will promote environmental burden reduction initiatives, such as chlorofluorocarbon-free and energy-saving design, contributing to achieving the Chugai Group’s Mid-Term Environmental Goals 2030. In addition, both buildings will adopt the digital infrastructure to support new operations of the production function, established and utilized in the Ukima Plant (project name: SPIRITS). In addition, initiatives to realize cost-competitive smart factories using robotics will be accelerated.
[Overview of Utsunomiya Plant]
- 1. Location:
- 16-3 Kiyohara-Kogyodanchi, Utsunomiya City, Tochigi Prefecture
- 2. Site area:
- 121,573 m2
- 3. Business activities:
- Bio-API production, formulation, inspection, and packaging of bio products
[Overview of construction plan of UT3]
- 1. Total investment:
- 37.4 billion yen
- 2. Start of construction:
- January 2024
- 3. Completion of building:
- May 2026
- 4. Start of operation:
- October 2026
- 5. Construction area:
- 3,206 m2 (4-story base isolated building)
- 6. Total floor area:
- 9,791 m2
- 7. Overview of facility:
- 2,000 L×4 single-use bioreactors + 1 purification line
[Overview of construction plan of UTA]
- 1. Total investment:
- 19.0 billion yen
- 2. Start of construction:
- January 2024
- 3. Completion of building:
- November 2025
- 4. Start of operation:
- March 2026
- 5. Construction area:
- 2,589 m2 (3-story base isolated building)
- 6. Total floor area:
- 7,682 m2
- 7. Overview of facility:
- Robot filler


[Image of Framework of Antibody API Production (Clinical Development to Initial Commercial Production)]
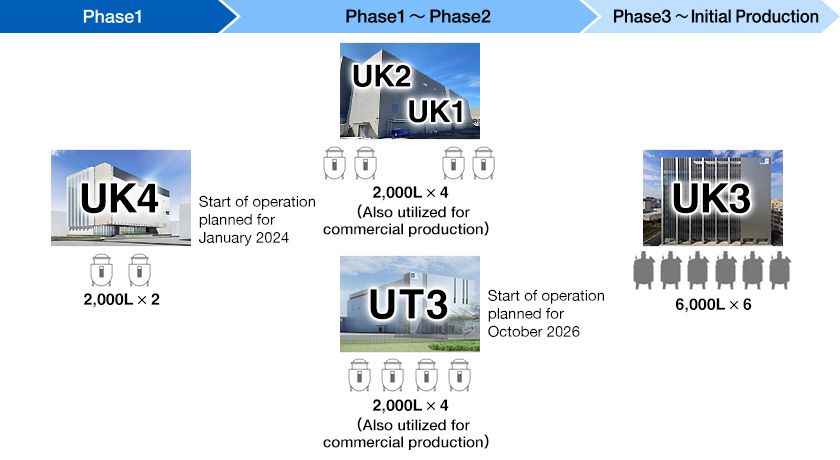
[Overview of Antibody API Production Facilities]
| Site | Building | Target | Bioreactors | Features |
|---|---|---|---|---|
| Ukima | UK1, UK2 | Commercial products/ Investigational drugs (Small-scale) | 2,000 L x 4 Single-use |
|
| UK3 | Commercial products/ Investigational drugs (Large-to -medium-scale) |
6,000 L x 6 Stainless steel tanks |
|
|
| UK4 (Under construction) | Investigational drugs (Small-scale) |
2,000 L x 2 Single-use |
|
|
| Utsunomiya | UT1 | Commercial products (Large-scale) |
10,000 L x 2 Stainless steel tanks |
|
| UT3 (New Building) | Commercial products/ Investigational drugs (Small-scale) |
|
|
Contact:
- For Media
- Chugai Pharmaceutical Co., Ltd.
- Media Relations Group, Corporate Communications Dept.,
- Toshiya Sasai
- Tel: +81-3-3273-0881
- E-mail: pr@chugai-pharm.co.jp
- For Investors
- Chugai Pharmaceutical Co., Ltd.
- Investor Relations Group, Corporate Communications Dept.,
- Takayuki Sakurai
- Tel: +81-3-3273-0554
- E-mail: ir@chugai-pharm.co.jp
